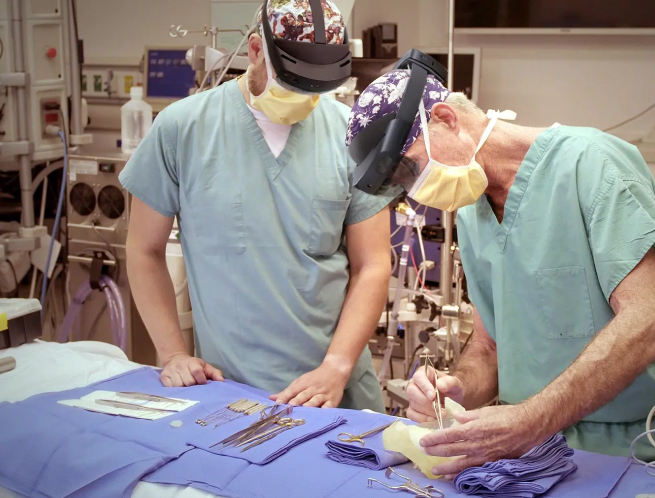
VSI HoloMedicine® allows visualization of capabilities for DICOM (MRI, PET, SPECT CT, and CBCT), OBJ, GLB, FBX and STL (3D models).
Find all product instructions, intended purpose, and proper use available in EN and DE here.
VSI is a software platform to be used by clinicians for the visualization of medical images in 3D to allow for surgical planning activities, patient education, and teaching, offering an intuitive and immersive user experience. When accessing VSI software from a wireless stereoscopic HMD or mobile device, images viewed are for information purposes only and not intended for diagnostic use, and users should rely on certified diagnostic tools for clinical decisions.
Find all product instructions, intended purpose, and proper use available in EN here.
VSI HoloMedicine® is a software device for displaying digital medical images acquired from CT, Angio CT, MRI,CBCT, PET, and SPECT sources. It is intended to visualize 3D imaging holograms of the patient for pre-operative planning outside and/or inside the surgical room. When accessing VSI HoloMedicine® from a wireless head-mounted display (HMD) or PC monitor, images viewed are for informational purposes only and not intended for diagnostic use. VSI HoloMedicine® is indicated for use by qualified healthcare professionals including surgeons, radiologists, physicians, and technologists.
Find all product instructions, intended purpose, and proper use available in EN and DE here.
*Please note, features & use cases in the USA are subject to the FDA 510(k) clearance certification.

Austria, Belgium, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malaysia, Malta, Netherlands, New Zeeland, Poland, Portugal, Romania, Singapore, Slovakia, Slovenia, Spain, Sweden, Turkey, UK, USA.
FDA 510(k) – Clearance (USA)
Class IIa – Registered (EU) CE
Class A – Approved (SG) HSA
Class I – Registered (UK) MHRA
Class I – Registered (NZ) WAND
Class A – Approved (MY) MDA